25. Is it possible to explore a time if you correctly scaled up a chemical procedure from lab scale to manufacturing scale?
A CQA is usually a Bodily, chemical, biological or microbiological property or attribute that needs to be in an proper limit, range, or distribution to be certain the specified merchandise top quality.
Furthermore, it features the details in the action executed by whom, checked by whom, at what time action was performed, at what date activity was performed and signature with the personnel linked to the batch or activity.
“I have in depth knowledge employing both HPLC and NMR gear. For the duration of my postgraduate scientific studies, I made use of these resources often for compound identification and quantification in advanced mixtures.
• Computer system-based or virtual simulations of certain unit functions or dynamics can offer method comprehending and assistance steer clear of troubles at business scale
The exam that's utilized to examine the integrity of packed strips, blisters, Bottles and modest sachets containing tablets, Capsules and Dry Powders is named leak test.
On this submit, I've bundled the complete task code, a description of the challenge, a code snippet of what I tried as well as the mistake messages I'm obtaining.
When these Preliminary tests display assure, Phase I scientific trials are performed on humans to further more Appraise protection. All through this process, it’s very important to constantly review and analyze information to make sure the drug’s security profile stays satisfactory.”
It's preserved for long term reference / reanalysis in conditions of market place grievances or improvement do the job or another clarification with regards to the produced batch.
Validation is the documented system that gives a higher diploma of assurance that a selected course of action, check here technique or system will continuously generate a consequence meeting predetermined acceptance requirements.
Significant: Grievances related to the product or service not meeting its pre-identified vital specs and damage to Principal packaging.
24. Precisely what is Calibration : The demonstration that a specific instrument or unit provides success inside specified limits by comparison with All those produced by a traceable typical about an correct choice of measurements.
Widespread Pharmaceutical Chemist get more info job interview questions, how to answer them, and illustration answers from a certified career coach.
And lastly, I participated in regular audits to recognize any regions of likely non-compliance and took immediate corrective action Every time important.”
 Kel Mitchell Then & Now!
Kel Mitchell Then & Now! Joseph Mazzello Then & Now!
Joseph Mazzello Then & Now!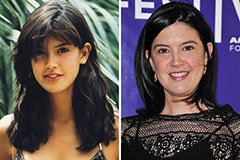 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Robbie Rist Then & Now!
Robbie Rist Then & Now! Terry Farrell Then & Now!
Terry Farrell Then & Now!